Clarithromycin Powder
Appearance: White Powder
CAS: 81103-11-9
MOQ: 10Kg
Package: 10Kg/Paper drum, 25Kg/Paper drum
Stock: 500 Kg
Delivery Time: Within 2~3 working days after you order
Payment Way: Bank Transfer, TT
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
Clarithromycin powder is a derivative of erythromycin, successfully developed by Japan's Taisho Company in the early 1990s, and registered under the trade name Clarith.Thereafter, Taizheng first transferred its technology to Abbott of the United States for production. It was launched in Ireland and Italy in 1990.It was approved by the FDA in October 1991 as a Class IB new drug.he trade name was Biaxin. In 1993, it was launched in Hong Kong, China as Klacid. Listed in many countries, the market usage has grown steadily, and it has played an important role in clinical practice. Clarithromycin and its tablets, currently produced dosage forms include granules, dispersible tablets, sustained-release tablets, injections and dry suspensions.
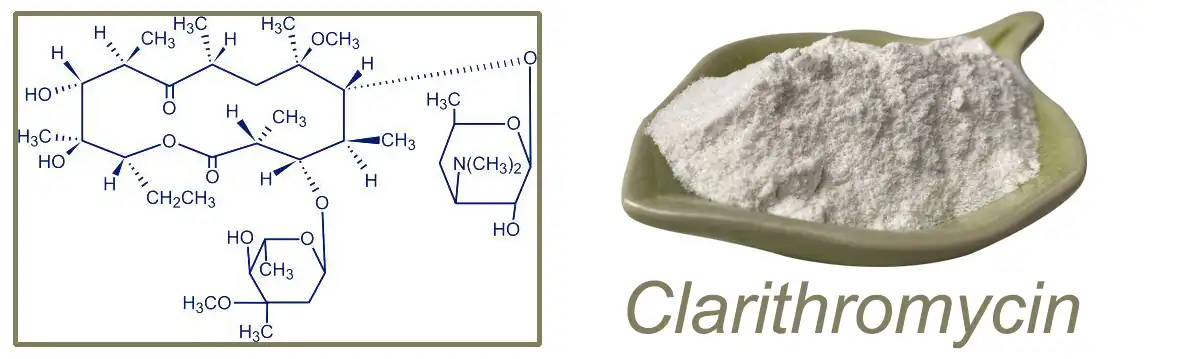
Basic Information:
Name | Clarithromycin |
CAS | 81103-11-9 |
Molecular Formula | C38H69NO13 |
Molecular Weight | 747.953 |
PSA | 182.91000 |
LogP | 2.43970 |
Stability | Cold Storage |
Adaptation Disease:

It is suitable for the following infections caused by clarithromycin-sensitive bacteria:
●Nasopharyngeal Infection: Tonsillitis, Pharyngitis, Sinusitis;
●Lower respiratory infection: Acute bronchitis, exacerbation of chronic bronchitis, and pneumonia;
●Skin and soft tissue infection: Impetigo, erysipelas, folliculitis, boils, and wound infections;
●Acute otitis media, mycoplasma pneumoniae pneumonia, urethritis and cervicitis caused by chlamydia trachomatis, etc;
●Clarithromycin powder used in legionella infection, or in combination with other drugs for the treatment of Mycobacterium avium infection and Helicobacter pylori infection.
Package:

10Kg/Paper drum, 25Kg/Paper drum;
Delivery:
We also delivery within 2~3 working days after you order.










